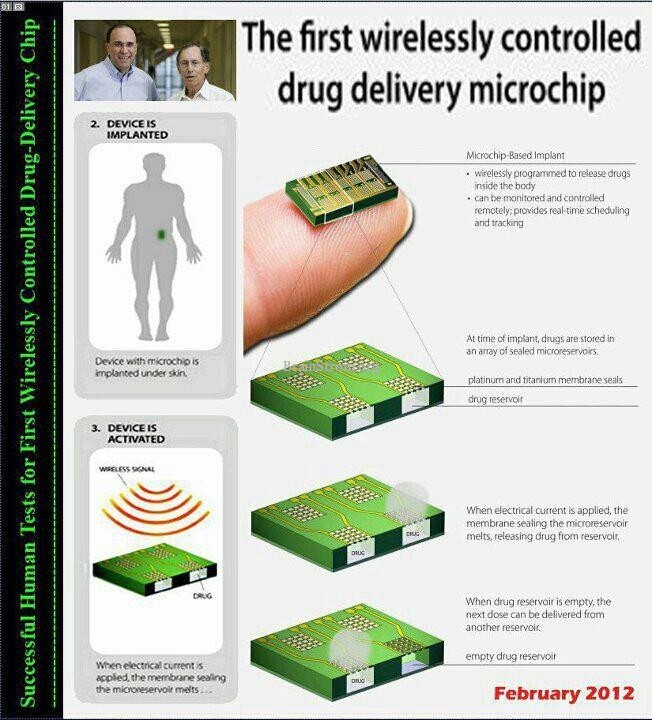
Scientists have conducted the first human trial of an implantable microchip-based drug delivery device, an assembly that releases precise doses of a drug through a wireless communication link and receives return messages confirming proper operation.
In cooperation with two commercial companies, scientists at Harvard, the Massachusetts Institute of Technology and Case Western Reserve University created a chip that holds measured doses of teriparatide (brand name Forteo), an injectable drug used to treat osteoporosis.
The cost of the drug delivered by the implant is about $10,000 to $12,000 a year, according Robert Farra, the lead author of an article describing the trial published on Thursday in the journal Science Translational Medicine. This is about the same as the cost of Forteo delivered by injection.
Dr. Ethel Siris, a professor of clinical medicine and director of the Osteoporosis Center at Columbia University Medical Center, said that Forteo was a very effective drug but difficult to administer, and that this kind of technology could prove useful.
“I can see many problems with the practical application,” she said. “But whether Forteo is the drug that’s going to get administered with this or some other kind of injectable, it’s pretty neat stuff.”
Approval of the device by the Food and Drug Administration is still some years away. The company plans to file for approval in 2014, Mr. Farra said, and even then at least two more years of clinical studies will be required before the device can be sold. Mr. Farra is president and chief executive of Microchips Inc., the company that is developing the device under license from M.I.T.
The chip is one-fiftieth of an inch thick and measures half an inch long by a fifth of an inch wide. It contains 10 cube-shaped reservoirs, 40 one-thousandths of an inch on each side. Each holds 600 nanoliters of a highly concentrated solution of the drug.
The side of the reservoirs that touches human tissue is covered with a metallic membrane, a composite of titanium and platinum, wired to internal electronics that provide a path for an electrical current. To deliver the drug, the membrane is melted.
The chip can be programmed to release the drugs on a schedule or activated by an operator using a computer when the patient is present. It uses the same frequency that all medical electronics use for communication.
Teriparatide is a particularly difficult drug to administer — daily injections are required — and this makes patient compliance an issue. The implanted chip may help eliminate the problem.
The assembly consists of two chips on the surface of a titanium housing that holds the electronics. The entire implant is about two and a quarter inches long and one and a half inches wide. For the trial, the researchers implanted the chips in seven women who had osteoporosis but were otherwise healthy.
Implanting the device is a fairly simple outpatient procedure. The patient, injected with a local anesthetic, gets a one-inch incision slightly below and to one side of the navel to create a pocket into which the device is fitted. The microchips are placed facing the muscle, and the entire device is sewn in place with two stitches. The incision is closed with a nylon suture.
The researchers waited eight weeks to allow the device to become stable in collagen tissue that the body develops in reaction to the foreign object. There was some question whether the drug could cross that fibrous membrane.
Then for the next month, they released up to 19 daily doses of the drug, either while the patient was under observation or automatically under the control of the programmed chip.
Blood tests showed that the drugs dispensed by microchip were as effective as ordinary injections in increasing bone mass and bone mineral density, and the individual automatic releases were slightly more consistent in quantity and effect than daily shots. There were no serious side effects.
Mr. Farra said that the trial “validates this approach to delivering drugs. It is safe and reliable. It assures compliance of the patient and improves outcomes.”
Thanks for installing the Bottom of every post plugin by Corey Salzano. Contact me if you need custom WordPress plugins or website design.